Device Overview
The use of anesthesia machines is integral to modern medicine, enabling safe and effective administration of anesthesia during surgical procedures and other medical interventions. These sophisticated medical devices have evolved significantly over the years.
The Anesthesia Machine is a critical medical device used to administer controlled doses of anesthesia to patients during surgical or medical procedures. It plays a pivotal role in ensuring patient comfort and safety during these interventions. Understanding the key components and operation of an Anesthesia Machine is essential for medical professionals and anesthetists who work with this device.
Novel Features
In the ever-advancing field of medical technology, anesthesia machines are continually evolving to offer novel features and improvements in patient care. Here are some of the novel features and advancements in modern anesthesia machines:
- Integrated Monitoring Systems: Many modern anesthesia machines come equipped with integrated patient monitoring systems. These systems provide real-time data on vital signs such as blood pressure, heart rate, oxygen saturation, and end-tidal carbon dioxide (EtCO2) levels. These features ensure anesthetists have access to critical patient information at their fingertips.
- Advanced Ventilation Modes: Anesthesia machines now offer a variety of ventilation modes, including pressure-controlled ventilation (PCV), volume-controlled ventilation (VCV), and pressure support ventilation (PSV). These modes allow for more precise control of a patient's respiratory parameters.
- Touchscreen Interfaces: Novel anesthesia machines often feature user-friendly touchscreen interfaces that enable anesthetists to make quick and accurate adjustments to settings. These interfaces provide visual feedback, making it easier to monitor and control the administration of anesthesia gases.
- Automated Record-Keeping: Some machines come with built-in electronic medical record (EMR) systems that allow for automatic recording of anesthesia data, reducing the need for manual charting. This feature streamlines documentation and improves accuracy.
- Remote Monitoring and Control: With the advent of telemedicine and remote healthcare, many anesthesia machines can be monitored and controlled remotely. This is particularly valuable for situations where the anesthetist may not be in close proximity to the patient.
- Low-Flow Anesthesia: Anesthesia machines with low-flow capabilities can conserve anesthetic gases, reducing the cost and environmental impact associated with gas consumption.
- Quick Gas Changeovers: Modern machines feature quick-change systems for switching between different gases, allowing for faster adaptation to changing patient needs.
- Safety Features: Enhanced safety features include high and low-pressure alarms, oxygen failure protection devices, and automatic ventilator failover mechanisms, all of which ensure the safety of the patient during anesthesia administration.
- Built-In Tutorials: Some machines offer built-in tutorials and educational materials, making them useful for training purposes and ensuring that healthcare providers can maximize their capabilities.
- Ergonomic Design: Novel anesthesia machines prioritize ergonomic design, making them more user-friendly and efficient for healthcare providers. Adjustable monitor arms, flexible breathing circuit configurations, and improved maneuverability are all part of this focus on ergonomics.
- Eco-Friendly Features: Many anesthesia machines now incorporate eco-friendly features such as low gas consumption, reduced waste gas emissions, and efficient power usage.
- Wireless Connectivity: Some advanced anesthesia machines offer wireless connectivity for seamless integration with hospital networks, enabling the transfer of real-time patient data and remote troubleshooting.
Regulatory Overview Anesthesia Machine
|
Risk Classification |
|
|
Type of Device |
Active Non-Implantable Device |
|
India |
Class C (Moderate High Risk) |
|
US FDA |
Class II (Medium Risk) |
|
EU Union |
Class IIb (Moderate High Risk) |
|
United Kingdom |
Class IIb (Moderate High Risk) |
|
Harmonized Standards |
|
|
ISO 13485 |
Quality management systems - Requirements for regulatory purposes |
|
ISO 14971 |
Application of risk management to medical devices |
|
ISO 20416 |
Post-market surveillance for manufacturers |
|
ISO 20417 |
Information to be supplied by the manufacturer |
|
ISO 80601-2-13 |
Medical electrical equipment - Part 2-13: Particular requirements for basic safety and essential performance of an anaesthetic workstation |
|
ISO 10993 |
Biological evaluation of medical devices |
|
Labeling and Labeling Requirements |
|
|
ISO 15223 |
Symbols to be used with information to be supplied by the manufacturer |
|
Chapter VI, MDR-2017 |
Labeling Requirements (India) |
|
Regulatory Pathways and Approvals |
|
|
India |
Manufacturing/Import/Loan License under Medical Device Rules 2017 |
|
Europe |
Conformité Européene (CE) Marking, Medical Device Regulation 2017/745 |
|
US FDA |
510(k) clearance, Premarket Approval (PMA) |
Clinical Evidence
Clinical evidence means, in relation to a medical device, the clinical data and the clinical evaluation report that supports the scientific validity and performance for its intended use.
Clinical Use
Anesthesia machines are essential tools in the field of healthcare, serving a critical role in ensuring the safety and comfort of patients during surgical and medical procedures. These versatile devices are used to induce various forms of anesthesia, provide respiratory support, and closely monitor patient parameters. Equipped with safety features and alarms, anesthesia machines guarantee patient well-being by maintaining secure airways, stable vital signs, and precise control over anesthetic agents and gas concentrations. Their adaptability for pediatric and adult patients, along with the ability to handle emergency situations, makes them indispensable in clinical settings. Anesthesia machines not only contribute to the success of medical procedures but also prioritize patient safety and the well-being of healthcare providers.
Clinical Evaluation of Anesthesia Machine
Clinical evaluation of anesthesia machines is a crucial process that ensures the safe and effective delivery of anesthesia during medical procedures. These evaluations involve rigorous testing, monitoring, and assessment of the machine's performance in real clinical settings. Clinical evaluation aims to confirm that the anesthesia machine functions as intended and adheres to safety standards.Clinical evaluation of anesthesia machines is an ongoing process, ensuring that these devices meet the highest standards of patient care and safety. By rigorously testing and assessing their performance in real clinical environments, healthcare institutions can provide the best possible care to patients undergoing surgical and medical procedures.
Safety and Evaluation of Anesthesia Machines
The safety and evaluation of anesthesia machines are of paramount importance in healthcare settings to ensure the well-being of patients during surgical and medical procedures. Anesthesia machines are complex devices designed to deliver precise concentrations of anesthetic gases, control ventilation, and monitor various physiological parameters.- Safety Standards and Regulations (Regulatory Approvals)
- Gas Safety and Handling (Leakage, Storage and Scavenging)
- Electrical Safety
- Operator Training (Proficiency and Identification of interference that may affect readings)
- Maintenance and Calibration
- Performance Evaluation (Accuracy and Signal Quality)
- Software Validation Report
Biocompatibility
Biocompatibility in anesthesia machines involves the careful selection of materials and rigorous testing to ensure patient safety. The use of biocompatible materials and adherence to regulatory standards are critical to prevent any harm or discomfort to patients during medical procedures involving anesthesia machines. Since no material directly comes in contact with the Patient or Patient’s Skin however accessories may have contact with the patient which may require undergo biological evaluation depending upon the contact with patient.Current State of the Art (SOTA) for Anesthesia Machine
The "Current State of the Art" (SOTA) in the context of medical devices like anesthesia machines refers to the most advanced and up-to-date technology, features, and design elements available in the field. It represents the cutting edge of innovation and showcases the highest standard of performance and patient safety.Design
The design of anesthesia machines is a critical factor in their overall functionality and usability. It aims to create a safe, user-friendly, and efficient device that meets the specific needs of healthcare professionals while ensuring the well-being of patients. Advances in design continue to drive improvements in anesthesia machine technology. Here are key design-related aspects of anesthesia machines:- Ergonomics: Anesthesia machines are designed with a focus on ergonomics. The layout and placement of controls, monitors, and gas delivery components are carefully considered to facilitate efficient operation. Anesthetists need quick and easy access to various settings, and the machine's design ensures they can make adjustments with minimal effort.
- Compact Form: Modern anesthesia machines feature a compact and space-saving design, making them suitable for a variety of clinical settings, including operating rooms and intensive care units where space can be limited. Portability is also a consideration for machines used in ambulances and during patient transport.
- Modular Construction: Many anesthesia machines have a modular design, which allows for flexibility and customization. Healthcare facilities can choose the specific modules and components that suit their needs, making it easier to upgrade or adapt the machine over time.
- Accessibility and Visibility: The design incorporates features that improve visibility and accessibility. This includes well-lit displays, large, clear screens, and easy-to-read data. Ventilation parameters, gas concentrations, and patient monitoring data should be easily visible to the anesthetist.
- Cable Management: Anesthesia machines are designed to manage cables and hoses effectively to prevent clutter and tripping hazards in the operating room. Well-designed cable management systems ensure a clean and organized workspace.
- Infection Control: The materials and surfaces used in the design should be resistant to cleaning agents and disinfectants. Smooth, non-porous surfaces are preferred to prevent the accumulation of contaminants and ensure thorough cleaning.
- Safety Features: The design incorporates numerous safety features, such as color-coded knobs and connectors to minimize the risk of errors, tamper-evident seals, and easy access to emergency shut-off controls. Mobility and Portability: Some anesthesia machines are designed with mobility in mind. They may include wheels for easy transport or built-in handles for lifting. Mobility is particularly important in emergency situations and during patient transfers.
Material of Construction
The materials used in the construction of anesthesia machines are carefully selected to ensure the safety, durability, and reliability of these critical medical devices. Here are the typical materials of construction used in anesthesia machines:- Stainless Steel: Used for the external casing, frames, and structural components due to its corrosion resistance and durability.
- Brass: Used for gas flow control valves and components due to its non-corrosive properties and ability to maintain gas purity.
- Aluminum: Often used for lightweight components and structures within the machine.
- Copper: Used for certain internal components and tubing in specific areas of the machine.
- Anodized Aluminum: Used for vaporizers due to its corrosion resistance and inertness with anesthetic agents.
- Medical-Grade PVC (Polyvinyl Chloride): Commonly used for tubing and hoses due to its flexibility, ease of cleaning, and compatibility with medical gases.
- Silicone Rubber: Used for seals, gaskets, and some tubing due to its biocompatibility and airtight sealing properties.
- High-Impact Plastics: Utilized for housing and casing for its strength and durability, as well as resistance to chemicals and cleaning agents.
- Polycarbonate: Occasionally used for transparent or shatter-resistant components.
- Silicone Rubber: Widely used for seals, gaskets, and O-rings due to its excellent biocompatibility and sealing properties.
- Rubber Compounds: Used in various areas of the machine for shock absorption and sealing purposes.
- Tempered Glass: Used for screens and display panels due to its durability and resistance to shattering.
- Filter Media: Typically made of specific filter materials designed to capture particles and moisture while allowing gas flow.
The specific materials used in the construction of devices can vary among manufacturers and models, depending on the device's intended use, design, and cost considerations. Manufacturers strive to balance the durability, functionality, and cost-effectiveness of these materials to provide reliable medical devices for healthcare professionals.
Manufacturing
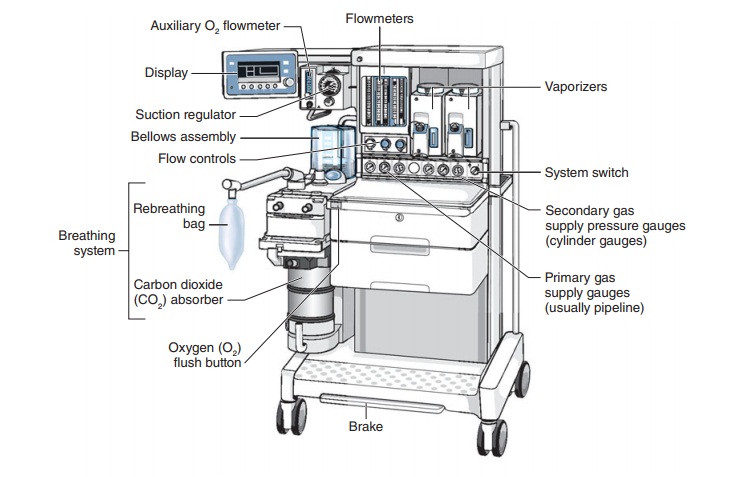
Manufacturers of anesthesia machines must adhere to strict quality management systems and regulatory requirements to ensure the safety and effectiveness of their devices. Continuous monitoring and improvement of the manufacturing process are essential to meet the demands of the healthcare industry and protect patient safety. Understanding the key components and operation of an Anesthesia Machine is essential for medical professionals and anesthetists who work with this device.- Gas Sources: An anesthesia machine is connected to various gas sources, including oxygen, nitrous oxide, and medical air. These gases are essential for administering the right mix of anesthesia to the patient.
- Flowmeters: Flowmeters are used to regulate and measure the flow of gases from the source to the patient. They are equipped with control knobs that allow anesthetists to adjust gas flow rates accurately.
- Vaporizers: Anesthesia vaporizers are responsible for converting liquid anesthesia agents into a vaporized form. These vaporized agents are then mixed with the carrier gases and delivered to the patient.
- Breathing Circuit: The breathing circuit consists of tubing and connectors that deliver the gas mixture from the machine to the patient. It includes the inspiratory limb (where gases flow from the machine to the patient) and the expiratory limb (where exhaled gases exit).
- Ventilator: Some anesthesia machines feature a ventilator, which helps in delivering controlled and regulated breaths to the patient. Modern machines often come with advanced ventilation modes and settings.
- Carbon Dioxide Absorber: To ensure that exhaled gases are free of carbon dioxide (CO2), an absorbent canister is included in the machine. This canister removes CO2 from the exhaled gases, making it safe to re-breathe for the patient.
- Pressure Gauges and Alarms: Pressure gauges and alarms are integrated into the machine to monitor and display the pressure levels of gases and breathing circuits. Alarms alert the anesthetist if there are any deviations from the safe parameters.
- Waste Gas Scavenging System: This system collects and removes excess anesthetic gases and exhaled gases from the operating room, preventing exposure to healthcare providers.
- Monitors: Many anesthesia machines are equipped with monitoring systems that display vital patient information, such as oxygen saturation (pulse oximetry), end-tidal carbon dioxide (EtCO2) levels, and anesthetic agent concentrations.
Principle of Operation
The operation of an Anesthesia Machine involves the following steps:- Gas Selection: Anesthetists select the appropriate gases required for the patient's procedure. This typically includes oxygen, nitrous oxide, and air.
- Gas Flow Control: The flowmeters are adjusted to control the flow rate of each gas precisely. The anesthetist sets the gas flow rates according to the patient's age, weight, and the type of procedure.
- Vaporizer Settings: If volatile liquid anesthetic agents are used, the vaporizer settings are adjusted to provide the desired concentration of the anesthetic agent in the gas mixture.
- Breathing Circuit Setup: The breathing circuit is connected to the patient, ensuring that the inspiratory and expiratory limbs are correctly aligned. It should provide a secure connection between the patient and the machine.
- Patient Monitoring: Vital signs, including heart rate, blood pressure, oxygen saturation, and EtCO2, are continuously monitored to ensure patient safety during anesthesia administration.
- Ventilation Management: If the anesthesia machine has a ventilator, the anesthetist can manually or automatically adjust ventilation settings to maintain the patient's respiratory parameters within safe ranges.
- Gas Scavenging: The waste gas scavenging system keeps the operating room free of excess anesthetic gases.
Software Information
Software plays a crucial role in modern anesthesia machines, contributing to their functionality, safety, and monitoring capabilities. Here's an overview of the software-related aspects in anesthesia machines:- Control and Monitoring Software: Anesthesia machines are equipped with sophisticated control software that allows anesthetists to precisely adjust parameters such as the concentration of anesthetic gases, flow rates, and ventilation settings. These software interfaces are typically user-friendly and intuitive, ensuring that healthcare professionals can make real-time adjustments during surgery.
- Integration with Patient Monitoring Systems: Many anesthesia machines have software interfaces that can seamlessly integrate with hospital or clinic-wide patient monitoring systems. This integration allows real-time monitoring of a patient's vital signs, such as heart rate, blood pressure, oxygen saturation, and end-tidal CO2 levels. This comprehensive data helps anesthetists make informed decisions during surgery.
- Alarm Systems: Software in anesthesia machines includes alarm systems that alert healthcare providers to abnormal conditions. For instance, if oxygen or anesthetic gas concentrations fall outside safe limits, alarms will activate, providing an early warning of potential issues.
- Data Logging and Documentation: Anesthesia machines often feature software that records and stores data from each procedure. This data can include ventilation settings, gas concentrations, and vital signs. This information is crucial for documenting the procedure, analyzing outcomes, and ensuring compliance with regulatory standards.
- Remote Monitoring and Telemetry: Some advanced anesthesia machines have software capabilities for remote monitoring and telemetry. This means that anesthetists and healthcare providers can monitor a patient's status and make adjustments even if they are not in the immediate vicinity, enhancing patient safety.
- Decision Support and Safety Checks: Software may include built-in decision support systems and safety checks. For instance, it can alert healthcare providers if the chosen combination of gas concentrations is potentially harmful or if there's a mismatch between the chosen ventilator settings and the patient's condition.
- Automatic Record-Keeping: Software can automate the record-keeping process, ensuring that all relevant data from the procedure is accurately and consistently documented. This feature helps in maintaining comprehensive patient records and streamlining administrative tasks.
- User Authentication and Access Control: Security is paramount in anesthesia machines' software. Access control measures and user authentication help prevent unauthorized personnel from making critical adjustments or accessing patient data.
Note: The Device Classification and applicable regulatory pathways may vary of deviate depending upon the features (Novel, multipara etc.) and interaction of the device have with patient or indication for use.


Thank you for sharing a clear and informative overview of anesthesia machines and their regulatory requirements. Understanding compliance is crucial for ensuring patient safety and device efficiency. For more insights on advanced medical solutions, For more info, Please Visit Us: https://sosaley.com/
ReplyDelete