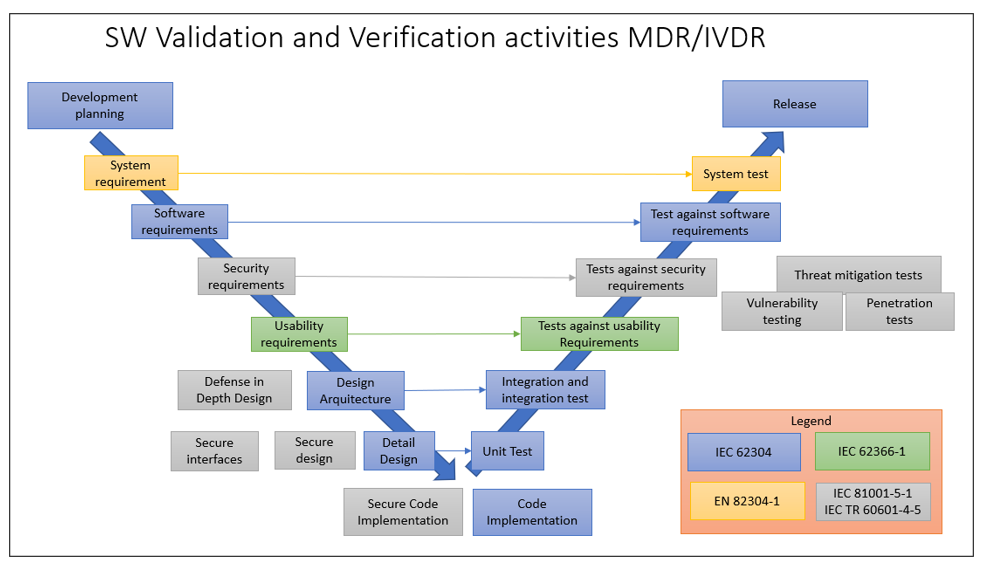
Verification and Validation (V&V) are critical components in the lifecycle of medical device software, ensuring that products meet their specifications and perform reliably and safely in their intended environments. Adherence to stringent regulatory requirements is essential to demonstrate compliance and to bring medical devices to market.
Disclaimer
Note: This for general guidance and understanding derived from various industry sources like ESA, and Software based companies.
Components of Software Verification and Validation
Objectives of V&V
- The primary goal is to confirm that the software products and processes comply with predefined standards, guidelines, specifications, and procedures.
Inspection Process
- Inspections involve systematic examination of software products to identify defects and ensure compliance with standards. Roles in the inspection team include moderators, readers, inspectors, and authors.
Formal Reviews
- Conducted to evaluate the correctness and completeness of the software through various techniques like technical reviews, walkthroughs, and software inspections.
Dynamic and Static Analysis
- Dynamic Analysis: Evaluating the behavior of software during execution.
- Static Analysis: Evaluating the software based on its structure, documentation, and form without executing it.
Integration Testing
- Testing combined software and hardware components to evaluate their interactions and ensure they work together as intended.
Regression Testing
- Selective retesting to ensure modifications do not introduce new faults and that the system remains compliant with its requirements.
Anomaly Reporting and Resolution
- Reporting and resolving discrepancies found during reviews using forms like the Review Item Discrepancy (RID) form.
Control Procedures
- Identifying configuration management procedures to ensure products of review, proof, and tracing are secure from accidental or deliberate alteration.
Standards and Practices
- Compliance with internal and external standards, practices, and conventions that govern review, proof, and tracing activities.
Reporting
- Documenting the results of V&V activities through various reports such as summary reports, technical review reports, and audit reports.
Regulatory Requirements
- Adherence to standards like ISO 13485 for Quality Management Systems (QMS) in medical devices.
- Following the ISO 14971 standard for risk management in medical devices.
- Using methodologies such as Failure Modes and Effects Analysis (FMEA) to identify and mitigate potential risks.
THE SOFTWARE VERIFICATION AND VALIDATION PLAN - Template 👇
SOFTWARE VALIDATION REPORT - Template 👇
Disclaimer
The information contained in this Software Verification and Validation Plan (SVVP) & Report is provided for guidance and informational purposes only. While every effort has been made to ensure the accuracy and completeness of the information provided, it is not intended to be a substitute for professional judgment and expertise.
This SVVP is based on the standards, guidelines, and regulatory requirements available at the time of its creation. It is the responsibility of the reader to ensure that they are using the most current and applicable standards and guidelines for their specific context.
The authors and publishers of this SVVP do not assume any liability or responsibility for errors or omissions in the information provided or for any outcomes related to the use or implementation of the plan. Users of this SVVP are advised to consult with qualified professionals and regulatory bodies to ensure compliance with all relevant requirements and to address any specific circumstances that may arise.
By using this SVVP, the reader agrees to assume full responsibility for any decisions or actions taken based on the information contained herein.