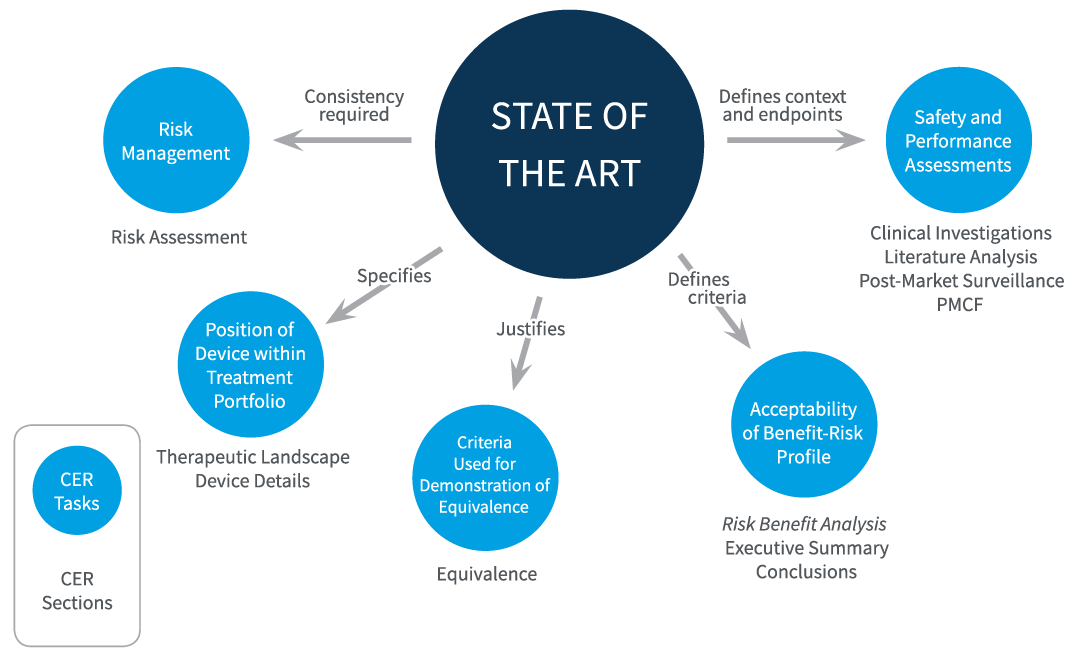
The State of the Art (SOTA) plays a crucial role in complying with the Medical Device Rules 2017, Medical Device Regulation (EU MDR) and In Vitro Diagnostic Regulation (EU IVDR). It is intricately linked to various aspects within technical documentation (TD) of the devices, including but not limited to clinical evaluation, scientific validity, risk management, and post-market surveillance. Feedback from readers confirms that regulatory/notified bodies prioritize the assessment of SOTA during TD reviews. They seek clarity on how SOTA is identified and updated, and whether it informs the assessment of the benefit-risk ratio. This article aims to elucidate the implications of SOTA for manufacturers of medical devices and in vitro diagnostics (IVDs).
How is SOTA defined for medical devices and IVDs?
Developed stage of current technical capability and/or accepted clinical practice in regard to products, processes and patient management, based on the relevant consolidated findings of science, technology and experience.
- The term "state-of-the-art" encapsulates the prevailing standards and practices deemed acceptable in technology and medicine at a given time. It's important to note that "state-of-the-art" doesn't always signify the most cutting-edge or advanced solution available. Instead, it refers to the commonly accepted norms and practices within a particular field. This interpretation of the state-of-the-art is sometimes referred to as the "generally acknowledged state-of-the-art."
- The state-of-the-art in the relevant medical domain encompasses the latest knowledge and advancements pertinent to the field. This includes the applicable standards and guidance documents governing the development and use of medical devices. Additionally, it involves a in depth understanding of the medical condition addressed by the device and its typical progression. Benchmark devices and alternative medical solutions available to the target population are also integral components of the state-of-the-art assessment.
- The concept of essential requirements (As per Medical Device Rules 2017) & General Safety and Performance Requirements (Annex-I of Medical Device Regulation and In-Vitro Diagnostic Regulation) is based on the assumption that the harmonised standards reflect generally acknowledgeable state of the art.
How does SOTA affect the product life cycle?
The state-of-the-art (SOTA) significantly influences the entire product life cycle of medical devices.
- Research and Development: During the initial stages of product development, SOTA guides research efforts by defining the current technological landscape and identifying gaps or areas for improvement. It helps researchers and developers stay abreast of the latest advancements in the field, ensuring that new devices incorporate state-of-the-art features and functionalities.
- Design and Prototyping: SOTA serves as a benchmark for designing and prototyping medical devices. Designers use current knowledge and best practices to create innovative and effective solutions that meet or exceed industry standards. Prototypes are developed with SOTA in mind to ensure they align with the latest technological advancements and address current clinical needs.
- Regulatory Compliance: Regulatory agencies require manufacturers to demonstrate that their medical devices meet current standards and incorporate state-of-the-art technologies. Compliance with SOTA is essential for obtaining regulatory approval or clearance. Manufacturers must stay updated on SOTA throughout the regulatory approval process to ensure their devices meet evolving standards and requirements.
- Manufacturing and Production: SOTA influences manufacturing processes by guiding the selection of materials, components, and manufacturing techniques. Manufacturers strive to incorporate the latest advancements in manufacturing technology to improve product quality, efficiency, and reliability. Continuous improvement initiatives are driven by SOTA to ensure products remain competitive in the market.
- Marketing and Sales: Products that incorporate state-of-the-art technologies and features have a competitive edge in the market. Marketing efforts highlight the innovative aspects of the product and emphasize its superiority over competing offerings. Sales teams leverage SOTA to showcase the device's capabilities and benefits to potential customers, emphasizing its alignment with current industry standards and practices.
- Post-Market Surveillance and Feedback: Even after a device is commercialized, SOTA continues to play a crucial role in its lifecycle. Post-market surveillance activities monitor the device's performance in real-world settings and identify opportunities for improvement based on emerging technologies and clinical practices. Customer feedback and market trends inform ongoing product development efforts to ensure the device remains state-of-the-art throughout its lifecycle.
- Development/design - Information about technology, material of construction,
- Manufacturing - The methods and machines required for manufacturing,
- Clinical evidence - Which clinical methods are established and have sufficient clinical data,
- Biological safety - Type and suitable material
Many manufacturers strive to incorporate state-of-the-art (SOTA) elements into their products, albeit sometimes indirectly. This integration often occurs through market analysis, literature research on technology advancements, competitor performance evaluations, or by aligning with diagnostic guidelines. However, in practice, we often observe that SOTA research is conducted at a later stage in the product lifecycle. For instance, manufacturers may commission SOTA research as part of a clinical evaluation or performance assessment, long after the product has been on the market. This delayed approach to SOTA integration can pose challenges, especially if the product's technical documentation lacks the necessary updates or fails to incorporate the latest advancements.
How SOTA should be integrated with Technical Documentation
Whereas manufacturers may encounter difficulties implementing SOTA findings into their technical documents due to various reasons such as resource constraints, limited access to updated information, or a lack of awareness regarding the importance of SOTA compliance. As a result, the product's documentation may not accurately reflect the current state-of-the-art, potentially impacting regulatory compliance, market competitiveness, and overall product performance.Integrating state-of-the-art (SOTA) information into technical documentation is essential for ensuring that medical devices and in vitro diagnostics (IVDs) meet regulatory requirements and industry standards while also reflecting the latest advancements in technology and medicine. Here's how SOTA should be integrated with technical documentation:
- Market Research: Conduct thorough market research to identify current trends, technological advancements, and emerging standards relevant to the product. This includes analyzing competitor products, customer needs, and regulatory expectations.
- Literature Review: Perform a comprehensive literature review to gather information from scientific publications, clinical studies, industry guidelines, and regulatory documents. This helps to stay updated on the latest research findings, best practices, and regulatory requirements applicable to the product.
- Regulatory Compliance: Ensure that the technical documentation complies with relevant regulations, standards, and guidelines governing medical devices and IVDs. This includes incorporating SOTA information related to safety, efficacy, performance, and quality management systems as required by regulatory authorities.
- Risk Management: Integrate SOTA insights into the risk management process to identify, assess, and mitigate potential risks associated with the product. This involves evaluating the latest clinical data, adverse event reports, and safety alerts to enhance risk assessment and mitigation strategies.
- Clinical/Performance Evaluation: Include SOTA evidence in the clinical evaluation process to demonstrate the safety, performance, and effectiveness of the product. This involves analyzing clinical literature, real-world data, and post-market surveillance findings to support the product's clinical claims and indications for use.
- Design and Development: Incorporate SOTA principles into the design and development process to ensure that the product incorporates the latest technological advancements and user requirements. This may involve iterative design reviews, usability testing, and validation studies to verify product performance against SOTA benchmarks.
- Documentation Updates: Regularly update the technical documentation to reflect changes in SOTA, regulatory requirements, and product modifications. This includes revising design inputs, risk assessments, labeling, and instructions for use to align with the current state-of-the-art.
If the product is on the market, SOTA should be updated regularly as part of the PMS activities. Define the research for the update of SOTA in your PMS plan – then analyze and evaluate the results in your PMS report or PSUR (depending on the product class). In terms of development/design and manufacturing, ask yourself, for example, if there are now materials or manufacturing processes available that are more suitable for your product. These aspects should also be considered in the further development of your products as well as in the development of follow-up products.