All IVD medical devices should meet accepted international quality and safety requirements. To formulate regulations for IVD medical devices, an understanding of the various standard-setting frameworks, the mechanisms used to determine standards and their use in conformity assessment is important. So, there is need to study the global aspects of IVD medical devices in ICH and selected countries, for better regulatory delivery.
The global in vitro diagnostics market size was valued at $71,496.16 million in 2020, and is projected to reach $106,914.16 million by 2030, growing at a CAGR of 4.08% from 2021 to 2030. In vitro diagnostics (IVD) include reagents, instruments, software use to evaluate specimens such as blood, urine, stool, tissues, and other bodily fluids obtained from the human body to diagnose diseases, conditions, and infections. Furthermore, there are several types of IVD devices that use diverse approaches such as diagnosticians, tissue diagnostics, haematological, and molecular diagnostics. The application and management of IVD market needs expertise technical skill; therefore, devices are employed in specialist medical facilities to perform diagnosis.
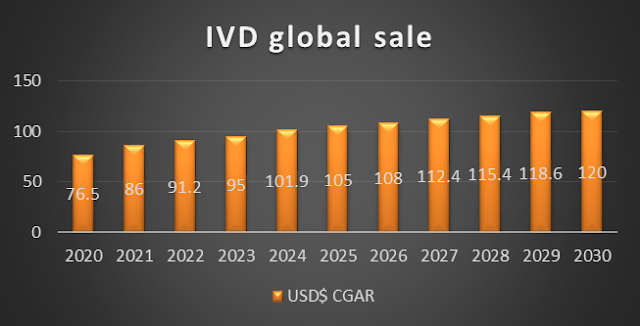 |
| Global IVD market size |
International Council for Harmonization (ICH)
The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) is unique in bringing together the regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of pharmaceuticals and develop ICH guidelines. Since its inception in 1990, ICH has gradually evolved, to respond to increasingly global developments in the pharmaceutical sector and these ICH guidelines are applied by a growing number of regulatory authorities. ICH's mission is to achieve greater harmonization worldwide to ensure that safe, effective and high-quality medicines are developed, and registered and maintained in the most resource efficient manner whilst meeting high standards.In Vitro Diagnostic medical device (IVD)
‘In Vitro Diagnostic (IVD) medical device’ means a medical device, whether used alone or in combination, intended by the manufacturer for the in-vitro examination of specimens derived from the human body solely or principally to provide information for diagnostic, monitoring or compatibility purposes.
In vitro diagnostic (IVD) devices encompass a wide range of products designed for diagnostic testing outside the human body. Some common types of IVD devices include:
- Clinical Chemistry Tests: Analyzing blood and bodily fluids for markers of diseases.
- Immunoassays: Detecting the presence of specific antibodies or antigens.
- Molecular Diagnostics: Identifying genetic material to diagnose infections or genetic disorders.
- Haematology Tests: Examining blood components like red and white blood cells.
- Coagulation Tests: Assessing the blood's ability to clot.
- Microbiology Tests: Identifying and characterizing microorganisms.
- Urinalysis Tests: Analyzing urine for signs of diseases.
- Point-of-Care Testing (POCT): Conducting tests at or near the site of patient care.
- Genetic Tests: Analyzing genes, chromosomes, or proteins for genetic conditions.
- Blood Glucose Monitoring: Measuring glucose levels for diabetes management.
Types of In-Vitro Diagnostic Devices
Within the vast landscape of in vitro diagnostics (IVD),
various categories cater to distinct purposes. This article describes the
complexities of the IVD domain, exploring the following categories:
Research Use Only (RUOs): Unlocking Scientific Frontiers
Research Use Only (RUO) in vitro diagnostics are purposefully crafted for research applications and are not intended for diagnostic or therapeutic purposes. These diagnostics occupy a pivotal role in advancing scientific understanding, serving as crucial assets in the initial phases of test development. Their primary objective is to contribute to the broader scientific knowledge base.
Investigational Use Only (IUOs): Pioneering Medical Product Development
Investigational Use Only (IUOs) come into play during the investigational phases of medical product development. Their purpose is to assist researchers and developers in gathering essential data, evaluating safety, and determining the efficacy of emerging diagnostics. IUOs are indispensable tools in the dynamic landscape of medical research.
Analyte Specific Reagents (ASRs): Precision in Detection
Analyte Specific Reagents (ASRs) constitute integral components within an IVD test system, specifically employed for the detection of a particular analyte. While not standalone tests, ASRs significantly contribute to the overall accuracy and precision of the diagnostic process, ensuring reliable and targeted results.
Lab Developed Tests (LDTs): Tailoring Diagnostics for Unique Needs
Lab Developed Tests (LDTs) are distinct in that they are conceived, developed, and executed within a single laboratory. Often tailored to address specific clinical needs, LDTs find common ground in academic and research institutions. These tests represent a flexible approach to diagnostics, allowing for customization to meet unique requirements.
Companion Diagnostic IVDs: Aligning Diagnostics with Therapeutics
Companion Diagnostic IVDs are purposefully designed to align with specific therapeutic products. Their role is crucial in identifying patients who are most likely to benefit from a particular treatment, ensuring a more targeted and personalized approach to healthcare.
In Vitro Diagnostic Multivariate Index Assay (IVDMIA): Unraveling Complexity for Diagnostic Insights
In Vitro Diagnostic Multivariate Index Assays (IVDMIAs) mark
a sophisticated category involving the measurement of multiple variables to
generate diagnostic results. These advanced assays analyze complex datasets,
providing valuable insights into various health conditions. IVDMIAs contribute
to a deeper understanding of intricate relationships within biological systems.
ESSENTIAL PRINCIPLES OF SAFETY AND PERFORMANCE FOR IVDS
(WHO Global Model
Regulatory Framework for Medical Devices including in vitro diagnostic medical
devices)
The Essential Principles for IVDs differ primarily in the following ways:
- Do not cover the incorporation of substances that are considered medicines because, even if they are present, they have no effect on the human body, because the mode of use of IVDs reduces the risk of infection with transmissible spongiform encephalopathy, place less emphasis on the requirement for veterinary controls on animals that are used as the source of biological material;
- Include a requirement in the design that the performance characteristics be used to support the intended use;
- Have fewer restrictions on electrical safety and energy supply because IVDs do not connect to the patient or supply energy to them;
- Include self-testing requirements for IVDs;
- Include criteria for the IVD's performance evaluation (whereas clinical evaluation is appropriate for medical devices that are not IVDs).
Clinical evidence for IVDs
In Vitro Diagnostics (IVDs), the foundation of credibility lies in robust clinical evidence, supported by scientific validity and impeccable analytical and clinical performance. This three-tiered approach is pivotal, ensuring that diagnostic tools not only meet regulatory standards but also deliver meaningful insights that resonate in clinical practice. All information that supports an IVD's scientific validity and performance when used as intended by the manufacturer is clinical evidence for an IVD. Along with other design verification and validation documentation, device description, labeling, risk analysis, and manufacturing information; it is a necessary part of an IVD's technical documentation for a manufacturer to demonstrate conformity with the Essential Principles.Data on analytical performance, clinical performance, and clinical validity make up clinical evidence. In relation to the collection of clinical data for IVDs, significant amount performance information received from performance analytics studies.
- Scientific Validity: A Bedrock of Trust
- Analytical Performance: Precision in Action
- Clinical Performance: Bridging the Gap to Practical Application
Why Clinical Evidence Matters:
- Assuring Safety: Robust clinical evidence assures both healthcare professionals and patients of the safety of the diagnostic tool, instilling confidence in its application.
- Informed Decision-Making: For healthcare providers, clinical evidence serves as a compass, guiding informed decision-making in diagnosis and treatment planning.
- Regulatory Compliance: Regulatory bodies require comprehensive clinical evidence to validate the efficacy and safety of IVDs, ensuring adherence to stringent standards.
- Patient-Centric Outcomes: Ultimately, the amalgamation of scientific validity and analytical and clinical performance translates into positive patient-centric outcomes. Reliable diagnostics contribute to accurate diagnoses, personalized treatment plans, and improved overall healthcare.


