Key components of a sterile barrier system typically include:
- Sterilization Wrap or Pouch: This is the outer layer of packaging that surrounds the medical device. It is constructed from materials that are compatible with the chosen sterilization method (such as steam or ethylene oxide).
- Seals and Closures: These are the mechanisms that seal the sterile barrier to prevent the ingress of microorganisms. Proper sealing is essential to maintaining sterility.
- Chemical Indicator: Many sterile barrier systems include a chemical indicator to show that the package has been exposed to the sterilization process. This provides a visual cue that the device inside should be sterile.
- Instructions for Use (IFU): The sterile barrier system may include instructions for the user regarding proper opening procedures to maintain sterility.
The selection of materials for sterile barrier systems in medical devices involves a comprehensive evaluation of various factors to ensure the effectiveness, safety, and regulatory compliance of the packaging. Here's an elaboration on each aspect:
- Microbial Barrier Properties: Materials must provide an effective barrier against microorganisms, preventing their ingress and maintaining the sterility of the medical device.
- Compatibility with the Device: The chosen materials should be compatible with the specific medical device they are intended to package, considering factors like size, shape, and surface characteristics.
- Biocompatibility / Toxicological: Materials must be biocompatible to avoid adverse reactions when the medical device comes into contact with biological tissues. Toxicological assessments ensure safety.
- Barrier Properties – Moisture, Gases, Light, etc.:The sterile barrier system should protect the device from external factors, including moisture, gases, and light, which could compromise the device's integrity.
- Physical / Chemical Properties (e.g., Porosity): Physical characteristics like porosity play a role in maintaining the integrity of the barrier. The material should resist tears, punctures, and other physical stresses.
- Method of Packing (e.g., Sealed, Folded, Taped, Need for Aseptic Opening):The packaging method should align with the requirements of the medical device and the sterilization process. Considerations include aseptic opening and user-friendly features.
- Material Limitations (e.g., Max Sterilization Temperature): Materials should withstand the sterilization process without compromising their properties. Understanding limitations, such as maximum sterilization temperatures, is crucial.
- Compatibility with Printing and Labeling Systems: Materials should support printing and labeling for clear identification and usage instructions. Compatibility ensures that information remains legible and intact.
- Storage and Transport Conditions: Materials should be selected based on the expected storage and transport conditions to ensure the sterility and integrity of the medical device are maintained.
- Environmental Aspects (e.g., Disposal, Recycling, Raw Material Consumption): Consideration of environmental aspects involves evaluating the material's impact on disposal, recycling requirements, and overall sustainability, in-cluding the consumption of raw materials and energy during production.
- Regulatory Compliance: Compliance with regulatory standards is paramount. Materials should meet the requirements of relevant regulations and standards governing sterile barrier systems for medical devices.
Sterilization – For Medical Devices
Sterilization is the process of effectively removing viable microorganisms, except prions, which are infectious agents primarily composed of proteins. It is a critical step to ensure the safety and efficacy of medical devices.Sterilization is a crucial process that aims to eliminate viable microorganisms, including spores, from surfaces, equipment, or articles, ensuring they are free from microbial contamination. However, it is practically impossible to prove the complete destruction of all organisms. To address this, Sterility Assurance Levels (SAL) serve as a measure of bioburden survival after terminal sterilization.
Key Points to understand the principles and objectives of Sterilization;
- Impossibility of Absolute Proof: Due to the practical impossibility of proving the complete elimination of all microorganisms, the focus shifts to achieving a high level of confidence in the sterility of the processed items.
- Sterility Assurance Levels (SAL): SAL is a measure expressed as a probability, representing the likelihood of viable microorganisms surviving after terminal sterilization. For instance, an SAL of 10-6 indicates a probability of less than or equal to one chance in a million that an item remains contaminated or non-sterile.
- Practical Application of SAL: SAL provides a practical way to assess the effectiveness of the sterilization process. The lower the SAL, the higher the level of sterility assurance, indicating a more stringent approach to microbial reduction.
- Risk Mitigation: SAL serves as a risk mitigation strategy by establishing an acceptable level of probability for microbial survival. It helps in defining the necessary measures and conditions required to achieve the desired level of sterility.
- Terminal Sterilization: SAL is particularly relevant in the context of terminal sterilization, which occurs after the final packaging of medical devices. This step is crucial in ensuring that the product reaches the end-user in a sterile condition.
- Continuous Monitoring: Achieving the desired SAL involves continuous monitoring, validation, and verification of the sterilization process. This includes using appropriate indicators and biological indicators to assess the effectiveness of the process.
- Regulatory Compliance: Meeting specific SAL requirements is often a regulatory necessity, and adherence to these standards ensures that medical devices comply with industry regulations and guidelines.
Sterilization Techniques
Terminal sterilization for medical devices can be achieved through a variety of techniques. No single method offers the perfect sterilization solution for every application. The main ones used in the medical device industry are as follows:Heat - Moist Heat (Steam), Dry Heat
Radiation – Beta respective Electron Beam, Gamma
Gaseous - Ethylene Oxide, Formaldehyde
Low Temperature Oxidative – Vaporized Hydrogen Peroxide (VHP), Hydrogen Peroxide Gas Plasma (VH2O2)
Chemical - Phthalaldehyde, sodium hypochlorite, hydrogen peroxide, glutaraldehyde, and peracetic acid
Porous materials are always required for the above processes except radiation, dry heat and the steam sterilization of aqueous liquids.
Heat Sterilisation
Moist heat is more effective than dry heat as it speeds up
heat penetration. Although the ultimate cause of microorganism death for both
moist and dry heat sterilisation is protein denaturation, it appears that moist
heat causes death of microorganisms by a slow burning process coagulating the
cell proteins, whereas dry heat is primarily a oxidative process. In the
absence of moisture, higher temperatures are required than when moisture is
present. However, moist heat cannot be used for hydrophilic materials.
Moist Heat (Steam)
Moist heat sterilisation is typically carried out in an
autoclave commonly using steam heated to 121–134 °C. To achieve sterility, a
holding time of at least 15 minutes at 121 °C (250 °F) or 3 minutes at 134 °C
is required.
Dry heat
Dry heat in the form of hot air is used primarily to
sterilise hydrophilic materials or materials that steam and ethylene oxide gas
cannot penetrate such as anhydrous oils, petroleum products, and bulk powders.
Typically used parameters for dry heat sterilisation are 2 hours holding time
at 160°C, but other temperatures up to 180°C can be used, for example 1 hour holding
time at 170°C or 30 minutes at 180°C. Although heating provides the most
reliable way to rid objects of all transmissible agents, steam and dry heat
sterilisation may be overly aggressive for device components or sterile barrier
materials and cannot be used for those that are heat or moisture sensitive.
Radiation Sterilisation
Gamma irradiation or electron-beam (e-beam or beta particle)
sterilisation are reliable alternatives for low temperature sterilisation, but
are generally only performed at a limited number of facilities due to the
higher investment costs involved. Sterilisation services using these methods
can be purchased on a contract basis. In these processes ionising radiation damages
micro-organisms by breaking chemical bonds and creating reactive free radicals and
ions. These species cause further chemical reactions within the cell disrupting
its function. Death of a micro-organism occurs by cumulative damage to the
cellular machinery, particularly the DNA molecule, thus preventing cellular
division and propagation of biologic life. Temperatures generated may still be
unsuitable for some materials with electron beam methods creating the most
heat. Irradiation can affect different polymers in different ways. Some effects
are detrimental and some are beneficial. The main effects observed are:
- Free radical initiation leading to polymer chain scission or cross linking. (Scission is the breaking of chemical bonds between atoms in the polymer chain. Cross links are bonds that link one polymer chain to another.)
- Change in average molecular weight
- Change in physical properties e.g. embrittlement
- Discolouration or gas or odour production
- Oxidation and time dependent effects
Gamma rays
Gamma rays are very penetrating and are commonly used for
sterilisation of disposable medical equipment, such as syringes, needles,
cannulas and IV sets. Cobalt 60 is a radioactive isotope capable of
disintegrating to produce gamma rays, which have the capability of penetrating
to a much greater distance than beta rays before losing their energy from
collision. Gamma radiation requires bulky shielding for the safety of the operators
and storage facilities for the Cobalt-60 which continuously emits gamma rays.
The product is exposed to radiation for 10 to 20 hours, depending on the
strength of the source.
Electron beam
Beta particles, free electrons, are transmitted through a
high-voltage electron beam from a linear accelerator. These high-energy free
electrons will penetrate into matter before being stopped by collisions with
other atoms. Thus, their usefulness in sterilising an object is limited by
density and thickness of the object. Although less penetrating than gamma rays,
electron beams are used as an on-off technology and provide a much higher
dosing rate than gamma rays. Due to the higher dose rate, less exposure time is
needed and thereby any potential degradation to polymers is reduced.
Gaseous
Ethylene Oxide
Ethylene oxide gas (EO or ETO) is also commonly used to
sterilise objects sensitive to temperatures greater than 60 °C such as plastics
or which are moisture sensitive. Ethylene oxide (ETO) is a chemical agent that
kills microorganisms, including spores, by interfering with the normal
metabolism of protein and reproductive processes (alkylation), resulting in death
of cells. Ethylene oxide treatment is generally carried out between 30 °C and
60 °C with relative humidity above 30% and a gas concentration between 200 and
800 mg/L. It takes longer than steam sterilisation, typically 16-18 hrs for a
complete cycle.
For ethylene oxide sterilisation it is essential that
materials are porous. Ethylene oxide penetrates well through porous materials
such as medical grade paper and polyolefin nonwoven materials and is highly
effective as a sterilant for sterile barrier systems which have adequate
porosity. However, ETO gas is highly flammable and toxic/carcinogenic so ETO sterilisation
is generally performed on a contract basis. Cycle times are relatively long, particularly
post-sterilisation because aeration is required to remove toxic residues.
Formaldehyde
Formaldehyde kills microorganisms by coagulation of protein
in cells. Used as a fumigant in gaseous form, formaldehyde sterilisation is
complex and less efficacious than other methods of sterilisation. It is used
only if other sterilisation methods are not available or are deemed unsuitable
for the item to be sterilised.
Low Temperature Oxidative
Hydrogen peroxide is used to sterilise heat or temperature
sensitive articles and materials. It is a strong oxidant and these oxidising
properties allow it to destroy a wide range of pathogens. In medical
sterilisation hydrogen peroxide is used at concentrations ranging from around
35% up to 90%. The biggest advantage of hydrogen peroxide as a sterilant is the
short cycle time. Whereas the cycle time for ethylene oxide (discussed above)
may be up to 18 hours, some modern hydrogen peroxide sterilisers have a cycle
time as short as 28 minutes.
Hydrogen peroxide is a strong oxidant and packaging
materials must be chosen to ensure compatibility. Cellulose based materials
such as paper products cannot be sterilised using hydrogen peroxide because it
reacts with the fibres. This weakens them and also means that there is little
if any peroxide left to act as a sterilant. Permeable polymer based materials such
as non-woven materials of polyolefin must therefore be used. The penetrating
ability of hydrogen peroxide is not as good as ethylene oxide and so there are
limitations on what can be effectively sterilised. The vapour is also hazardous
with the target organs being the eyes and respiratory system.
Vaporised Hydrogen Peroxide (VHP)
This method uses hydrogen peroxide vapour under vacuum to
sterilise medical devices. VHP technology demonstrates low toxicity and rapidly
decomposes into non-toxic by-products of water vapour and oxygen. Once the
vapour has been removed from the sterilisation chamber by a series of
vacuum/air pulses, unlike other processes such as ethylene oxide, no further
aeration is required.
Hydrogen Peroxide Gas Plasma
This technology uses a combination of hydrogen peroxide
vapour and low temperature gas plasma. After the hydrogen peroxide has
sterilised the devices and materials, an electromagnetic field is created in
which the hydrogen peroxide breaks apart producing a low temperature cloud that
contains ultra violet light and free radicals. Following the reaction the
activated components lose their high energy and recombine to form oxygen and
water. There is no need for aeration or cool down.
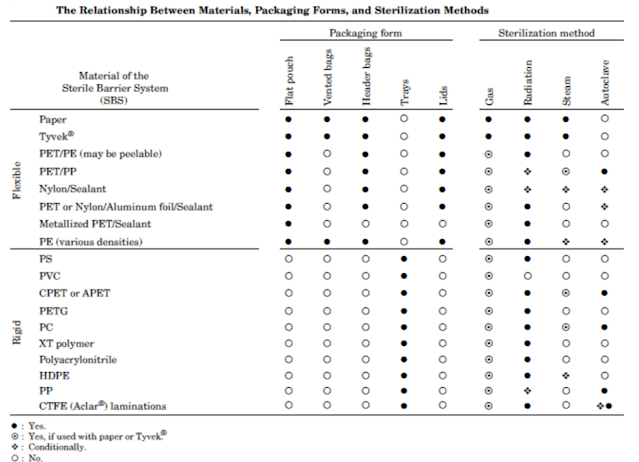
Choice of materials As Sterile Barrier System
Selecting the right materials for sterile barrier systems is a critical step in ensuring the integrity of medical devices and preventing contamination. Various factors come into play when making this decision, each contributing to the overall effectiveness of the sterile barrier. The tables below give some guidelines on material compatibility with the various sterilisation processes but it is important that the medical device manufacturer follows the sterile barrier manufacturer’s recommendations in selecting suitable sterile barrier systems for their particular products and processes.|
Material |
Permeability sufficient for steam and gaseous sterilization methods |
STEAM at least a part of the packaging needs to be permeable to steam |
EO/FORM at least a part of the packaging needs to be permeable to gas |
Hydrogen Peroxide (Plasma) natural fiber based materials are incompatible |
Gamma/E-Beam or Beta radiation impermeable material may be used |
Dry Heat (max temp) impermeable material may be used |
|
Medical grade
paper |
Y |
Y |
Y |
N |
Y |
Y (160°C) |
|
Flush spunbond nonwoven materials of polyethylene |
Y |
Y (max. T 127°C) not suitable for hospitals |
Y |
Y |
Y |
N |
|
Wet laid non-wovens (pulp and plastic fibres) |
Y |
Y |
Y |
N |
Y |
N |
|
SMS (Spunbond Meltblown Spunbond) nonwoven materials of polypropylene |
Y |
Y |
Y |
Y |
N |
N |
Films and composite films
|
Material |
STEAM at least a part of the packaging needs to be permeable to steam |
EO/FORM at least a part of the packaging needs to be permeable to gas |
Hydrogen Peroxide (Plasma) natural fiber based materials are incompatible |
Gamma/E-Beam or Beta radiation impermeable material may be used |
Dry Heat (max temp) impermeable material may be used |
|
Laminated films, widely used for the manufacture of
prefabricated sterile barrier systems (pouches, reels), impermeable |
|||||
|
PET/PP films (PET/Polypropylene) |
Y |
Y |
Y |
N |
N |
|
PET/PE films (PET/Polyethylene) |
N |
Y |
Y |
Y |
N |
|
Film components, blister materials, high barrier
composites, impermeable |
|||||
|
Aluminium laminates and composites, i.e. high barrier materials |
Y |
Y |
Refer Supplier
Specification |
Y |
Y |
|
APET film (Amorphous Polyethylene Terephthlate) |
N |
Y |
Refer Supplier
Specification |
Y |
N |
|
E/P (Ethylene-Propylene Copolymer) |
Refer Supplier
Specification |
Y |
Refer Supplier
Specification |
Refer Supplier
Specification |
Refer Supplier
Specification |
|
HDPE film (High Density Polyethylene) |
Y (121°C) |
Y |
Refer Supplier
Specification |
Y |
N |
|
LDPE film Low Density Polyethylene |
N |
Y |
Y |
Y |
N |
|
PA film (component) (Polyamide) |
Y |
Y |
Y |
Y |
Y |
|
PE film (component) (Polyethylene) |
N |
Y |
Y |
Y |
N |
|
PP film (component) (Polypropylene) |
Y |
Y |
Y |
N |
N |
|
PET film (component) (Polyethylene Teraphthalete) |
Y |
Y |
Y |
Y |
Y |
|
PETG (PETG-Foam, PETG-PE) film (PET Glycol) |
N |
Y |
Refer Supplier
Specification |
Y |
N |
|
PS film (Polystyrene) |
N |
Y |
Refer Supplier
Specification |
Y |
N |
|
HIPS film (High Impact Polystyrene) |
N |
Y |
Refer Supplier
Specification |
Y |
N |
|
PC film (Polycarbonate) |
Y |
Y |
Refer Supplier
Specification |
Y |
Y |
|
PVC film (Poly Vinyl Chloride) |
N |
Y |
Refer Supplier
Specification |
N |
N |
|
TPU film (Thermoplastic Polyurethane) |
N |
Y |
Refer Supplier
Specification |
Y |
Y |