An adverse event is any undesirable experience associated with the use of a medical product in a patient. Adverse events can range from mild to severe and can be life-threatening. Each year, the FDA receives several hundred thousand medical device reports (MDRs) of suspected device-associated deaths, serious injuries and malfunctions.
- An event that user facilities become aware of that reasonably suggests that a device has or may have caused or contributed to a death or serious injury or
- An event that manufacturers or importers become aware of that reasonably suggests that the device may have caused or contributed to a death or serious injury, or malfunction of the device.\
- Death
- Life-threatening or serious injury
- Hospitalization (initial or prolonged)
- Disability or Permanent Damage
- Congenital Anomaly/Birth Defect
- Required Intervention to Prevent Permanent Impairment or Damage (Devices)
- Other Serious (Important Medical Events)
The FDA uses MDRs to monitor device performance, detect potential device-related safety issues, and contribute to benefit-risk assessments of these products. These reports help us to protect the public health by helping to ensure that devices are not adulterated or misbranded and are safe and effective for their intended use.
21 CFR Part 803 is a regulation established by the Food and Drug Administration (FDA) that outlines the requirements for medical device reporting (MDR). The primary purpose of this regulation is to ensure that manufacturers, importers, and device user facilities promptly report adverse events and product problems associated with medical devices to the FDA.
Adverse Event Reporting Database
MedWatch
MedWatch is a FDA Safety Information and Adverse Event Reporting Program for health professionals, patients and consumers. MedWatch is the Food and Drug Administration's (FDA) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure, and product use errors with human medical products.
Through MedWatch, the FDA collects and evaluates reports submitted by the public, enabling swift action to address emerging safety concerns and prevent potential risks to public health. Reports received through MedWatch are carefully reviewed by regulatory experts, who analyze the data to identify trends, patterns, and potential safety issues associated with specific medical products.
In addition to receiving reports, MedWatch also disseminates safety alerts and advisories to healthcare professionals and the public, providing timely information about emerging risks and necessary actions to mitigate them. These alerts play a crucial role in facilitating communication between the FDA, healthcare providers, and patients, fostering transparency and trust in the regulatory process.
- Prescription and over-the-counter medicines
- Biologics such as blood components, blood/plasma derivatives and gene therapies.
- Medical devices such as hearing aids, breast pumps, and pacemakers.
- Combination products such as pre-filled drug syringe, metered-dose inhalers and nasal spray.
- Special nutritional products such as dietary supplements, medical foods and infant formulas.
- Cosmetics such as moisturizers, makeup, shampoos, hair dyes and tattoos.
- Food such as beverages and ingredients added to foods.
- Mandatory Reporting: Uses 3500A form
- Voluntary Reporting: Uses 3500 Form and 3500B form
Mandatory Reporting
The Medical Device Reporting (MDR) regulation (21 CFR Part 803) contains mandatory requirements for manufacturers, importers, and device user facilities to report certain device-related adverse events and product problems to the FDA. Form FDA 3500A is used for mandatory reporting of medical device adverse events by manufacturers, user facilities and importers.
| Reporter | When and What to Report | Whom to report | Form Number |
| Manufacturer | Within 30 calendar days of becoming aware of an event like deaths, serious injuries and malfunctions | FDA | Form FDA 3500A |
| Manufacturer | Within 5 work days of becoming aware of an event that requires remedial action to prevent an unreasonable risk of substantial harm to the public health | FDA | Form FDA 3500A |
| Importer | Within 30 calendar days of becoming aware of an event like deaths, serious injuries | FDA and the manufacturer | Form FDA 3500A |
| Importer | Within 30 calendar days of becoming aware of an event like malfunctions | Manufacturer | Form FDA 3500A |
| User Facility | Within 10 work days of becoming aware of device related death or serious injury | FDA and the manufacturer | Form FDA 3500A |
Voluntary Reporting
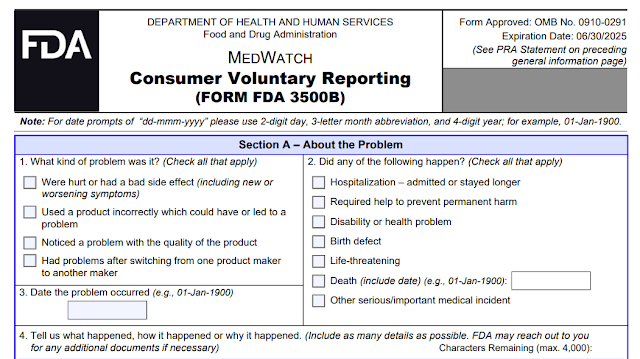
The FDA encourages healthcare professionals, patients, caregivers and consumers to submit voluntary reports of significant adverse events or product problems with medical products to Medwatch. There are 2 types of forms namely:- Form FDA 3500 may be used by health professionals or consumers for VOLUNTARY reporting of adverse events
- Form FDA 3500B is a consumer-friendly version of Form FDA 3500
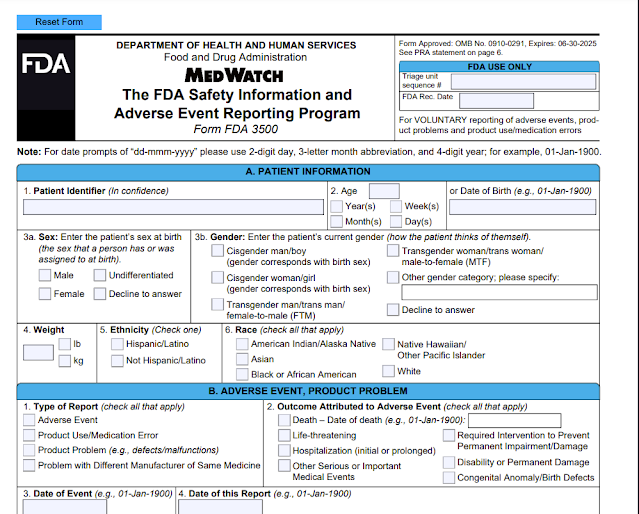 |
Form 3500 for Voluntary Reporting Medical Device Adverse Event Databases |
Medical Product Safety Network (MedSun)
MedSun, short for the Medical Product Safety Network, is a program developed by the U.S. Food and Drug Administration (FDA) to collect and analyze real-world data about the safety and performance of medical devices. The program aims to enhance patient safety by facilitating the early detection and investigation of potential issues related to medical devices used in healthcare settings.
MedSun operates as a network of healthcare facilities, including hospitals, ambulatory surgical centers, and nursing homes, across the United States. Participating facilities collaborate with the FDA to report adverse events, product problems, and near misses associated with medical devices used in their clinical practice. These reports provide valuable insights into the performance of medical devices in real-world settings and help identify emerging safety concerns.
The data collected through MedSun is used by the FDA to identify trends, patterns, and potential safety issues related to specific medical devices or device categories. This information enables the FDA to take appropriate regulatory action, such as issuing safety alerts, recalls, or guidance documents, to mitigate risks and protect patient safety.
Healthcare facilities participating in MedSun receive access to training, resources, and support from the FDA to facilitate reporting and enhance patient safety initiatives within their organizations. The collaborative nature of MedSun encourages healthcare professionals to actively engage in the reporting process and contribute to the continuous improvement of medical device safety.
Manufacturer and User Facility Device Experience (MAUDE)
MAUDE is a publicly accessible database that contains reports of adverse events, product problems, and device malfunctions submitted to the FDA by manufacturers, importers, and device users (such as healthcare providers and consumers). It serves as a valuable resource for monitoring device performance and identifying potential safety issues. The MAUDE database houses MDRs submitted to the FDA by mandatory reporters 1 (manufacturers, importers and device user facilities) and voluntary reporters such as health care professionals, patients and consumers. “MAUDE data is not intended to be used either to evaluate rates of adverse events or to compare adverse event occurrence rates across devices.” The MAUDE database contains mandatory reports filed by manufacturers and importers from August 1996 to present.
TPLC - Total Product Life Cycle database of US FDA
The Total Product Life Cycle (TPLC) database of the US Food and Drug Administration (FDA) is a repository of information related to medical devices regulated by the FDA. This database serves as a central hub for storing and managing data pertaining to various aspects of medical devices throughout their lifecycle, from premarket review to post-market surveillance and beyond.
Key features of the TPLC database include:
- Premarket Submissions: The TPLC database contains information on premarket submissions for medical devices, including 510(k) clearance applications, premarket approval (PMA) applications, investigational device exemptions (IDEs), and De Novo requests. These submissions undergo review by the FDA to assess the safety and effectiveness of the device before it can be marketed.
- Regulatory Actions: Information on regulatory actions taken by the FDA related to medical devices is documented in the TPLC database. This includes enforcement actions such as recalls, market withdrawals, warning letters, and other measures taken to address safety concerns or regulatory violations.
- Adverse Events Reporting: The TPLC database includes reports of adverse events associated with medical devices, submitted to the FDA through the MedWatch program or other reporting mechanisms. These reports provide valuable insights into potential safety issues or problems with device performance that may require further investigation or action by the FDA.
- Post-Market Surveillance: Data on post-market surveillance activities, including post-market studies, inspections, and audits conducted by the FDA, are captured in the TPLC database. These activities help monitor the ongoing safety and effectiveness of medical devices once they are on the market.
- Product Registrations and Listings: Manufacturers are required to register their medical devices with the FDA and provide listings of their marketed products. The TPLC database contains information on registered establishments, device listings, and other administrative details related to device registration and listing.
- Device Classification and Standards: Information on the classification of medical devices and applicable regulatory standards is available in the TPLC database. This includes device classification information under the FDA's risk-based classification system, as well as recognized consensus standards and guidance documents relevant to device regulation.
Application and benefits of Databases for Product Development
Databases play a critical role in streamlining product development processes, ensuring regulatory compliance, and delivering high-quality products that meet customer needs and expectations. By leveraging databases effectively, organizations can enhance efficiency, reduce time-to-market, and drive innovation in product development.- Market Surveillance Records
- List of adverse events and their reason/safety concern
- Inclusion in Preliminary Hazard Analysis in Risk Management
- Highlight all the residual risks in Technical Files