In the ever-evolving landscape of healthcare technology, software plays an increasingly integral role in the functionality and performance of medical devices and In Vitro Diagnostic (IVD) tools. Understanding the regulatory requirements, functionalities, and challenges associated with software in these devices is paramount for manufacturers, regulatory professionals, and healthcare practitioners alike.
Regulatory Landscape
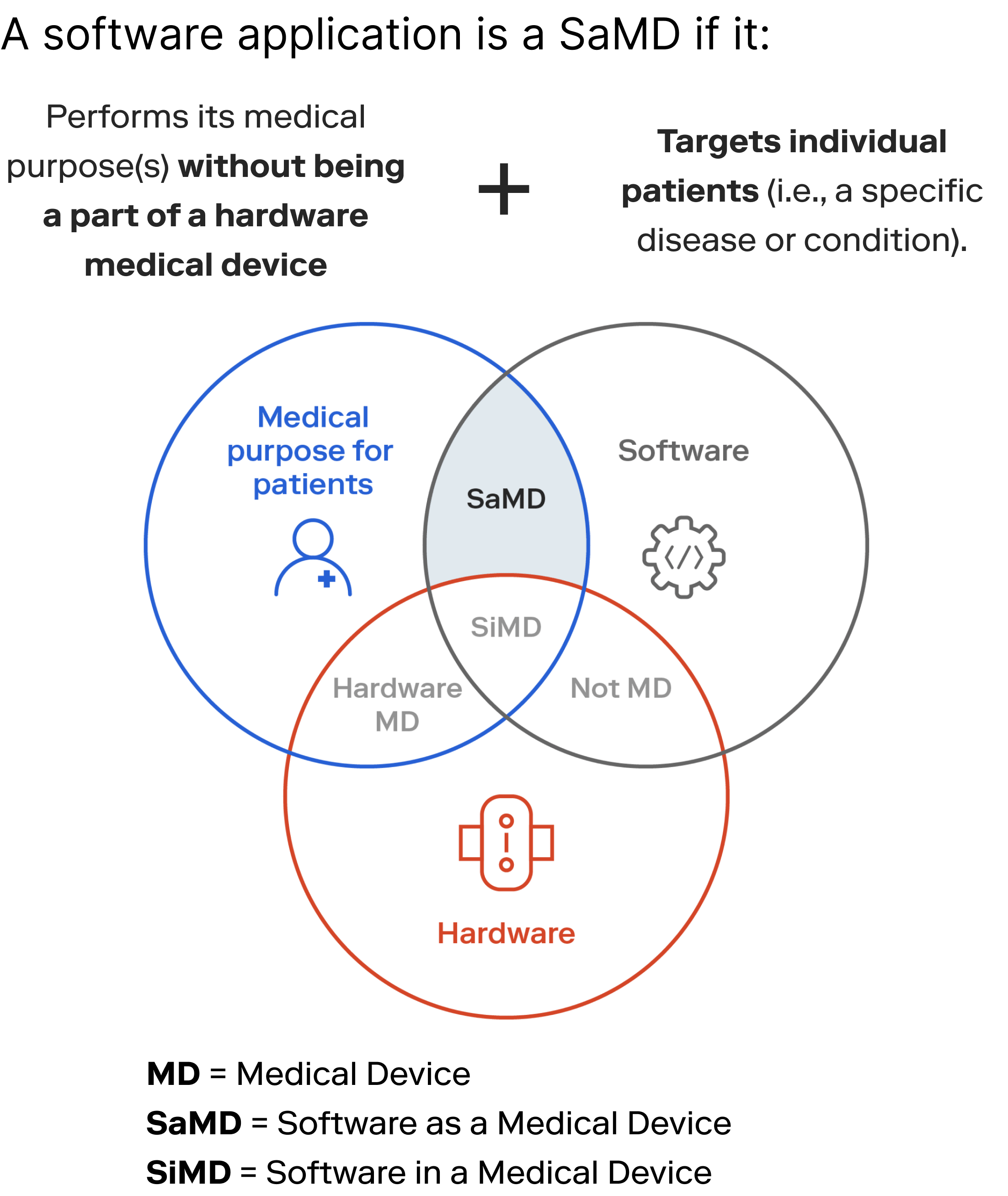
Regulatory oversight of software in medical devices and IVDs is governed by frameworks such as the FDA's Software as a Medical Device (SaMD) guidance and the European Union's Medical Device Regulation (MDR) & In Vitro Diagnostic Regulation (IVDR), Medical Device Rules India. Classification of software-based devices depends on factors such as risk level, intended use, and mode of action, with corresponding regulatory requirements ensuring patient safety and product quality.
From diagnostic imaging to patient monitoring and disease management, software-driven functionalities in medical devices and IVDs offer a wide range of clinical applications. These functionalities include data analysis, image processing, decision support, remote monitoring, and more. Real-world examples demonstrate the clinical benefits and improved patient outcomes facilitated by innovative software features.
Types of Software in Medical Devices and IVDs:
- Embedded Software: Embedded software refers to programs that are embedded directly into the hardware of medical devices or IVDs. This type of software controls the device's basic functions and operations, such as data acquisition, signal processing, and user interface interactions. Embedded software is tightly integrated with the device's hardware and typically operates in real-time to ensure smooth and efficient device performance.
- Standalone Applications: Standalone applications are software programs that operate independently of specific hardware devices. These applications can be installed on standard computing platforms such as desktop computers, laptops, or tablets. In the context of medical devices and IVDs, standalone applications are often used for data analysis, diagnostic interpretation, and remote monitoring. They offer flexibility and versatility, allowing healthcare professionals to access and analyze data from multiple sources conveniently.
- Software as a Service (SaaS) Solutions: Software as a Service (SaaS) solutions involve the delivery of software applications over the internet on a subscription basis. In the realm of medical devices and IVDs, SaaS solutions offer cloud-based platforms for data storage, analysis, and collaboration. These solutions enable healthcare organizations to access advanced analytics tools, share data securely, and collaborate with colleagues in real-time, regardless of geographical location. SaaS solutions also facilitate seamless software updates and maintenance, ensuring that users always have access to the latest features and improvements.
- Mobile Applications: Mobile applications, or apps, are software programs designed specifically for use on mobile devices such as smartphones and tablets. In the context of healthcare, mobile applications offer a wide range of functionalities, including patient monitoring, medication management, telehealth consultations, and health tracking. Mobile apps empower patients to take control of their health and enable healthcare providers to deliver personalized care anytime, anywhere. With features such as push notifications, real-time data synchronization, and integration with wearable devices, mobile applications enhance engagement and improve health outcomes for users.
- Artificial Intelligence (AI) Algorithms: Artificial intelligence (AI) algorithms leverage advanced computational techniques to analyze complex data sets, identify patterns, and make predictions or recommendations. In medical devices and IVDs, AI algorithms are used for tasks such as image recognition, diagnostic decision support, predictive analytics, and personalized treatment planning. By harnessing the power of machine learning and deep learning algorithms, AI-enabled devices can enhance diagnostic accuracy, improve treatment outcomes, and streamline clinical workflows. AI algorithms continuously learn from new data inputs, enabling them to adapt and evolve over time to meet the evolving needs of healthcare providers and patients.
The General Standrads
Two ISO standards are of high importance for software medical devices: ISO 13485 and ISO 14971. They can be seen as the topmost standards for medical devices. They are very generic and apply to every medical device, from the simplest plaster to the most complex surgical robot. ISO 13485 sets the stage for a robust quality management system, ensuring that organizations adhere to stringent standards throughout the device lifecycle. On the other hand, ISO 14971 provides indispensable guidance on risk management processes, indispensable for identifying hazards and implementing effective risk control measures.
Specific standards
These specific standards complement ISO 13485 and ISO 14971 by providing detailed requirements and guidance tailored to the unique aspects of software development, cybersecurity, and usability engineering in medical devices. Compliance with these standards is essential for ensuring the safety, effectiveness, and regulatory compliance of software medical devices.
- IEC 62304: It is a specific standard that addresses software lifecycle processes for medical device software. It outlines requirements for the development, maintenance, and risk management of software used in medical devices. IEC 62304 covers activities such as software development planning, requirements analysis, architectural design, implementation, verification, validation, and maintenance. Compliance with IEC 62304 ensures that medical device software is developed and maintained in a safe and effective manner.
- IEC 60601-1: It is a series of international standards that specify safety and essential performance requirements for medical electrical equipment. Part 1 of this series, IEC 60601-1, applies to embedded software within hardware medical devices. It addresses aspects such as electrical safety, electromagnetic compatibility, and environmental testing to ensure the safety and reliability of medical electrical equipment.
- IEC 82304-1: It is a standard specifically applicable to standalone software used as medical devices, also known as Software as a Medical Device (SaMD). It provides requirements and guidance for the development, validation, and lifecycle management of SaMD. IEC 82304-1 focuses on ensuring the safety, effectiveness, and performance of standalone software intended for medical purposes.
- IEC 81001-5-1: It is a standard that addresses cybersecurity requirements for medical device networks and systems. It provides guidance on identifying cybersecurity risks, implementing cybersecurity controls, and managing cybersecurity throughout the lifecycle of medical devices. Compliance with IEC 81001-5-1 helps mitigate cybersecurity threats and ensures the integrity and confidentiality of medical device data.
- IEC 62366-1: It is a standard that addresses usability engineering for medical devices. It provides guidance on applying human factors and usability engineering principles to the design and evaluation of the man-machine interface of medical devices. Compliance with IEC 62366-1 helps ensure that medical devices are safe, effective, and easy to use for intended users.
| Standard | Purpose | Responsible | Technical Expert |
| ISO 13485 | Quality System for medical devices industry | Quality Manager. | Software project manager: |
| ISO 14971 | Risk Management for medical devices | Quality Manager. | Software project manager |
| IEC 62304 | Software lifecycle for medical devices | Software project manager. | Quality Manager |
| IEC 81001‑5‑1 | Cybersecurity in medical devices | Software project manager and software security specialist | Quality Manager |
| IEC 60601‑1 | Programmable electrical medical systems (PEMS) in medical devices | Software project manager (for section 14) | Quality Manager |
| IEC 82304‑1 | Software as medical devices (SaMD) | Software project manager | Quality Manager |
| IEC 62366‑1 | Usability in medical devices | Software project manager and usability engineering specialist | Quality Manager |