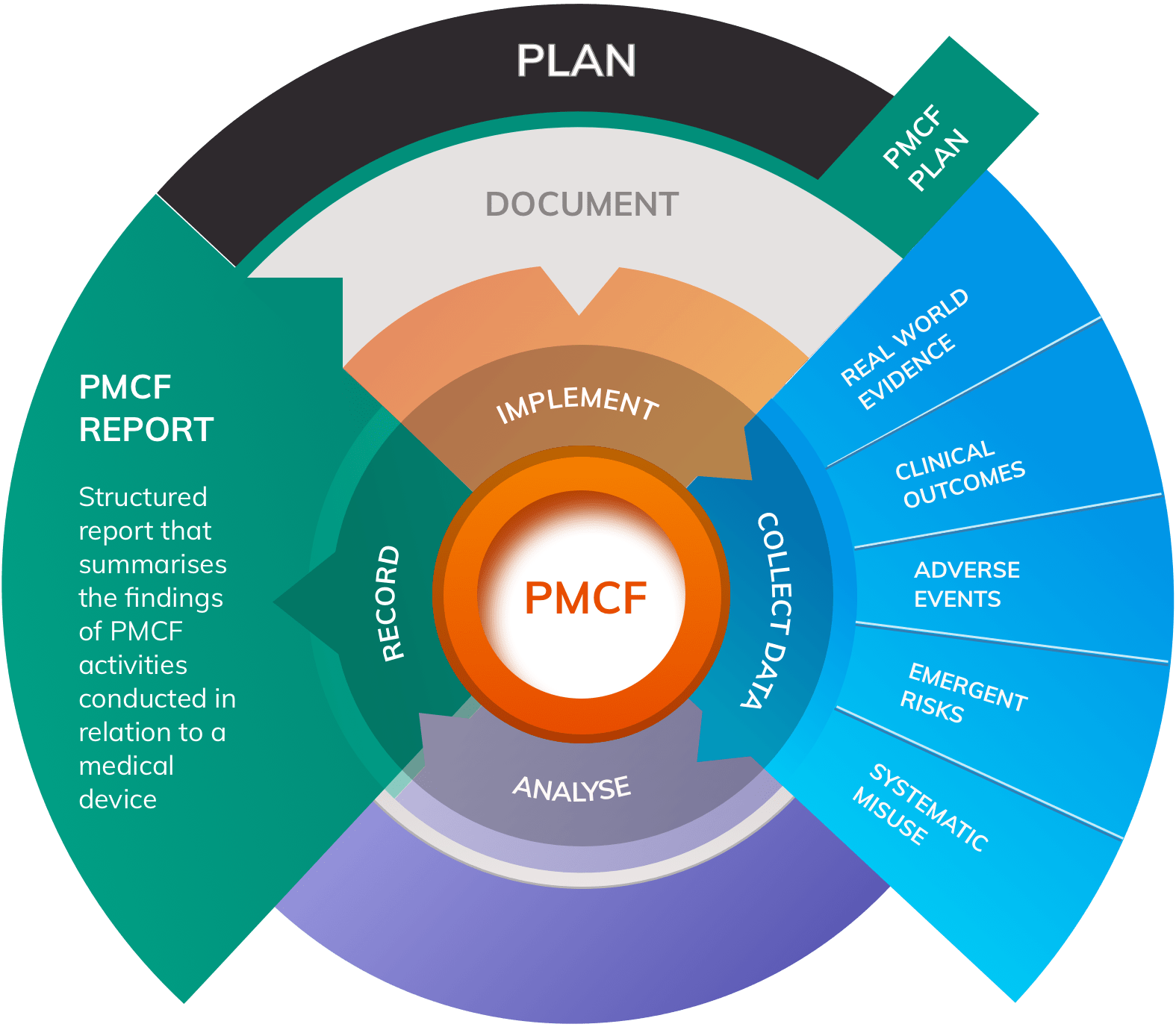
The field of medical devices is constantly evolving, with new technologies and innovations being introduced to improve patient outcomes. However, ensuring the safety and performance of these devices is crucial, which is where Post-Market Clinical Follow-up (PMCF) studies come into play. PMCF is an integral part of Post-Market Surveillance (PMS) and is mandated by the European Union Medical Device Regulation (EU MDR). In this article, we will explore the significance of the MDCG guidelines, specifically MDCG 2020-7, in conducting effective PMCF studies.
Real World Evidence (RWE) plays a crucial role in meeting the requirements of PMCF under the MDR. RWE captures data from a study or survey population that represents the entire population normally exposed to the device. It focuses on the safety and performance of the subject device without making comparisons between different devices. RWE studies the use of a CE-marked device and aims to confirm its safety and performance requirements.
PMCF studies and surveys can generate RWE of the required standard. The most versatile and powerful design is a PMCF study, while a PMCF survey can be effective and cost-efficient for lower-risk or consumer devices.
PMCF always returns data, but a Vigilance system can have no data if the device works flawlessly and results in no complaints, adverse events, or serious incidents. Both systems are crucial for ensuring the safety and performance of medical devices.
These MDCG guidelines provide stakeholders with a common understanding of how the MDR and IVDR should be applied in practice. They assist manufacturers, notified bodies, and other stakeholders in complying with the regulations, including the requirements for PMCF and PMS.
ISO 14155:2020, Guidelines for Good Clinical Practice for Medical Devices (ICHGCP), and Good Clinical Data Management Practices (GCDMP) are also valuable resources for ensuring standardized and high-quality PMCF studies.
Understanding PMCF and its Objectives
PMCF is a proactive process that involves collecting and evaluating clinical data on the safety and performance of a medical device in its intended use. It is an ongoing process that should be conducted throughout the entire lifetime of the device. The objectives of PMCF, as outlined in the MDR Annex XIV Part B, include:- Identifying and investigating residual risks associated with device use.
- Contributing to the update of Clinical Evaluation.
- Detecting emerging risks and previously unknown side-effects.
- Confirming the overall safety and performance of the device.
- Identifying systematic misuse of the device and its impact on safety and performance.
Design Considerations for PMCF under the MDR
Designing an effective PMCF system requires a strategic approach, considering the specific characteristics, target patient and user population, risk classification, and complexity of the device. While traditional clinical investigations may collect data for a limited time and on a limited patient population, PMCF requires longitudinal data collection throughout the device's lifetime.Real World Evidence (RWE) plays a crucial role in meeting the requirements of PMCF under the MDR. RWE captures data from a study or survey population that represents the entire population normally exposed to the device. It focuses on the safety and performance of the subject device without making comparisons between different devices. RWE studies the use of a CE-marked device and aims to confirm its safety and performance requirements.
PMCF studies and surveys can generate RWE of the required standard. The most versatile and powerful design is a PMCF study, while a PMCF survey can be effective and cost-efficient for lower-risk or consumer devices.
Documentation and Considerations for PMCF Clinical Investigations
PMCF clinical investigations, including medical device registries and surveys, must adhere to all legislative requirements related to medical research. Specific documents required for PMCF investigations include:- Clinical Investigation Plan (CIP) or protocol: Outlining the conduct of the PMCF study.
- Investigator's Brochure: Specifying the responsibilities of the principal investigator at each clinical site.
- Patient information leaflet and consent form: Ensuring patients are fully informed and documenting the process of informed consent.
Developing a PMCF Plan with MDCG Guidelines
Creating a suitable PMCF Plan requires a combination of regulatory experience, clinical expertise, and familiarity with relevant legislation and guidelines. While the MDR Annex XIV Part B outlines the substantive requirements of PMCF systems, additional guidance can be found in MDCG 2020-7 and other supporting guidelines. MDCG 2020-7 provides a template for producing MDR-compliant PMCF Plans and offers guidance on structuring PMCF objectives to ensure MDR compliance. The MedDev guidelines, such as MedDev 2.12/2 rev 2, can also assist in designing a PMCF strategy. It is important to note that PMCF Plans should be developed by experts in the field to ensure suitability for purpose and compliance with MDR requirements.The Relationship Between PMCF and PMS
While PMCF is an essential component of PMS, it is essential to understand that they are distinct systems with different objectives. PMS is a device-specific system that oversees quality across all company activities. It consists of a PMCF system, which proactively collects data on device performance, and a Vigilance system, which gathers information on complaints, and adverse events, and handles field safety corrective actions.PMCF always returns data, but a Vigilance system can have no data if the device works flawlessly and results in no complaints, adverse events, or serious incidents. Both systems are crucial for ensuring the safety and performance of medical devices.
The Role of MDCG in Guiding PMCF and PMS
The Medical Device Coordination Group (MDCG) plays a significant role in providing guidance and support for the implementation of the MDR and In Vitro Diagnostic Medical Device Regulation (IVDR). The MDCG endorses various documents, including guidelines, questions and answers, and position papers, to facilitate a harmonized and effective implementation of the regulations.These MDCG guidelines provide stakeholders with a common understanding of how the MDR and IVDR should be applied in practice. They assist manufacturers, notified bodies, and other stakeholders in complying with the regulations, including the requirements for PMCF and PMS.
Standardization and Harmonization in PMCF
Standardization is crucial in ensuring consistency and reliability in PMCF studies. The MDCG guides standardization for medical devices, emphasizing the importance of harmonized practices and alternative technical solutions. This promotes a unified approach to PMCF, ensuring that studies produce reliable and comparable data.ISO 14155:2020, Guidelines for Good Clinical Practice for Medical Devices (ICHGCP), and Good Clinical Data Management Practices (GCDMP) are also valuable resources for ensuring standardized and high-quality PMCF studies.
The Role of Unique Device Identifier (UDI) in PMCF
The Unique Device Identifier (UDI) system plays a significant role in PMCF by enabling the identification, traceability, and monitoring of medical devices throughout their lifecycle. The UDI system provides essential data for PMCF studies and facilitates the collection and analysis of device-specific data.As a Manufacturer, Why You Need CROs Help.
PMCF studies are essential for evaluating the safety and performance of medical devices in real-world settings. The MDCG guidelines, particularly MDCG 2020-7, provide valuable guidance for designing and conducting effective PMCF studies. By following these guidelines and ensuring compliance with the MDR, manufacturers can collect reliable clinical data, assess risks, and confirm the overall safety and performance of their medical devices. With the support of MDCG and adherence to standardized practices, the field of medical devices can continue to advance, prioritizing patient safety and improving healthcare outcomes. CROs are a leading organization in the field of medical device PMCF studies, providing expertise and support to manufacturers in complying with MDR requirements. With their knowledge and experience,they ensure the successful implementation of PMCF strategies and the generation of high-quality clinical evidence.Author.: NagaChandra Bhardwaj Padakandla
Tags
EUMDR