In the dynamic and highly regulated business landscape, organizations strive to ensure the highest level of quality in their products, services, and processes. To achieve this, they rely on robust Quality Management Systems (QMS) and the internationally recognized family of standards that govern them. The QMS family standards provide organizations with a structured framework to establish, implement, and continually improve their quality management practices, enabling them to meet customer expectations, comply with regulatory requirements, and drive organizational excellence.
The QMS family standards encompass a range of internationally accepted benchmarks that address different aspects of quality management. These standards serve as valuable tools for organizations across various industries, providing guidance, best practices, and a common language for quality management. By adopting these standards, organizations can enhance their operational efficiency, optimize resource utilization, reduce risks, and consistently deliver high-quality products and services.
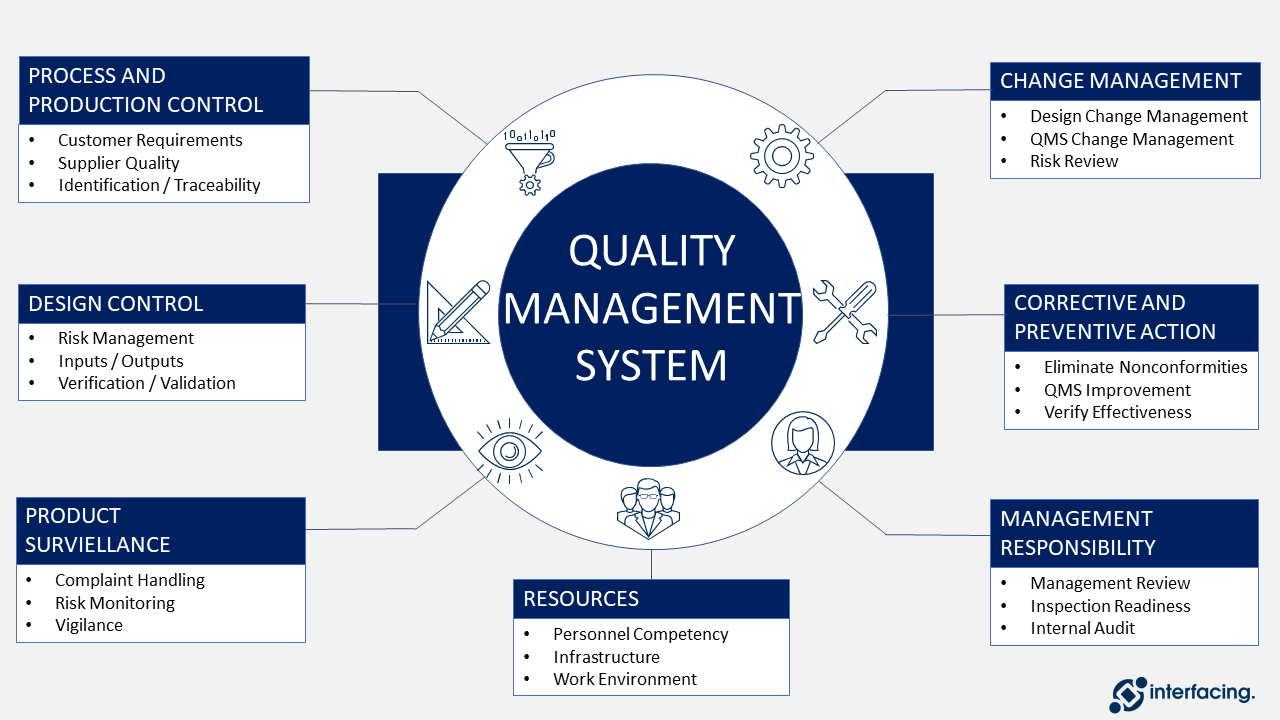 |
| Framework of Quality Management System |
ISO 9000: The Foundation of Quality Management
At the core of the QMS family standards lies ISO 9000, a foundational standard that sets the stage for organizations to establish and maintain effective quality management systems. ISO 9000 introduces the fundamental principles of quality management, including customer focus, leadership commitment, process approach, and continuous improvement. It provides a common language and understanding of quality management principles that serve as a solid foundation for organizations to build upon.
ISO 9001:2015: Achieving Certification for Quality Excellence
ISO 9001:2015 is the most widely recognized member of the QMS family and outlines the requirements for a quality management system that organizations can seek certification against. This standard provides a structured approach to quality management, encompassing areas such as leadership, planning, support, operation, performance evaluation, and improvement. By implementing ISO 9001:2015, organizations demonstrate their commitment to delivering products and services that meet customer requirements, enhancing customer satisfaction, and driving continual improvement throughout their operations.
ISO 13485: Elevating Quality Management for Medical Devices
For organizations operating in the medical device industry, ISO 13485 holds significant importance. This standard focuses specifically on the unique regulatory requirements and quality management needs of medical device manufacturers. ISO 13485 provides a comprehensive framework for the design, development, production, installation, and servicing of medical devices. Compliance with ISO 13485 ensures that organizations adhere to strict quality standards, prioritize patient safety, and maintain regulatory compliance in a highly regulated industry.
ISO 14001: Managing Environmental Responsibilities
In an era of growing environmental consciousness, ISO 14001 addresses the need for organizations to manage their environmental responsibilities effectively. This standard provides a systematic framework for establishing an Environmental Management System (EMS) that enables organizations to identify and control their environmental impact, reduce resource consumption, and comply with relevant environmental regulations. By integrating ISO 14001 into their QMS, organizations can demonstrate their commitment to sustainable practices and meet stakeholder expectations for environmental stewardship.
ISO 45001: Ensuring Occupational Health and Safety
The well-being of employees is a top priority for organizations, and ISO 45001 focuses on ensuring effective occupational health and safety management. This standard provides a framework for organizations to establish an Occupational Health and Safety Management System (OH&SMS) to protect the physical and mental well-being of their workforce. By adopting ISO 45001, organizations can identify hazards, assess risks, implement controls, and continually improve their safety performance, thereby creating a safe and healthy work environment.
The Power of Quality Management Systems
Quality Management Systems are the cornerstone of a company's commitment to consistently deliver safe, effective, and reliable medical devices. By implementing a comprehensive QMS, organizations establish a framework that encompasses processes, procedures, and policies for quality control, risk management, documentation, and continuous improvement. It serves as a guiding compass, aligning all aspects of the organization towards a unified goal: ensuring patient well-being and product integrity.
The Pillars of a Robust QMS
A robust QMS comprises several interconnected pillars that form the backbone of quality assurance. These include:- Document Control: Establishing clear guidelines for document creation, review, approval, and storage to ensure accurate and up-to-date information.
- Risk Management: Employing proactive measures to identify, assess, and mitigate risks throughout the product lifecycle, safeguarding against potential hazards.
- Training and Competency: Providing comprehensive training programs to equip employees with the necessary skills and knowledge to perform their roles effectively and in compliance with regulations.
- Supplier and Vendor Management: Implementing robust processes to assess, select, and monitor suppliers and vendors, ensuring the procurement of high-quality components and materials.
- Corrective and Preventive Actions: Instituting mechanisms to identify, investigate, and address non-conformities and implementing preventive measures to avoid recurrence.
- Customer Feedback: Collecting and Interacting with end user for Continuous Improvement
Continuous Improvement and Innovation
Embracing a culture of continuous improvement is at the heart of a successful QMS. By fostering an environment that encourages innovation, organizations can identify areas for enhancement, optimize processes, and proactively address emerging challenges. Incorporating feedback from customers, monitoring key performance indicators, and embracing new technologies are essential in driving efficiency, efficacy, and competitive advantage.
The QMS family standards serve as a powerful toolkit for organizations to achieve effective quality management, drive continuous improvement, and meet regulatory requirements. From the foundational principles of ISO 9000 to the certification framework of ISO 9001:2015 and the industry-specific focus of ISO 13485, these standards provide a solid foundation for organizations to build their quality management systems. Additionally, ISO 14001 and ISO 45001 expand the scope of QMS by addressing environmental sustainability and occupational health and safety, respectively. By adopting and integrating these QMS family standards, organizations can enhance their reputation, gain a competitive edge, and ensure long-term success by delivering high-quality products and services while prioritizing customer satisfaction, sustainability, and the well-being of their workforce.

